---
author: Sam White
toc-title: Contents
toc-depth: 5
toc-location: left
date: 2015-11-14 00:27:24+00:00
layout: post
slug: pcr-oly-rad-seq-prep-scale-pcr-2
title: PCR – Oly RAD-seq Prep Scale PCR
categories:
- 2015
- 2bRAD Library Tests for Sequencing at Genewiz
- Olympia oyster reciprocal transplant
tags:
- barcodes
- gel
- ILL-BC1
- ILL-BC10
- ILL-BC2
- ILL-BC3
- ILL-BC4
- ILL-BC5
- ILL-BC6
- ILL-BC7
- ILL-BC8
- ILL-BC9
- ILL-HT1
- olympia oyster
- Ostrea lurida
- PCR
- PTC-200
- Q5 High-Fidelity DNA Polymerase
- RAD
- RAD-seq
---
Continuing with the RAD-seq library prep. [Following the Meyer Lab 2bRAD protocol](https://github.com/sr320/LabDocs/blob/master/protocols/External_Protocols/2bRAD_11Aug2015.pdf).
[After determining the minimum number of PCR cycles to run to generate a visible, 166bp band on a gel yesterday](https://robertslab.github.io/sams-notebook/posts/2015/2015-11-13-pcr-oly-rad-seq-test-scale-pcr-4/), ran a full library “prep scale” PCR.
| **REAGENT**
|
**SINGLE REACTION (μL)**
|
**x11**
|
| Template
|
40
|
NA
|
| ILL-HT1 (1μM)
|
5
|
55
|
| ILL-BC# (1μM)
|
5
|
NA
|
| NanoPure H2O
|
5
|
55
|
| dNTPs (1mM)
|
20
|
220
|
| ILL-LIB1 (10μM)
|
2
|
22
|
| ILL-LIB2 (10μM)
|
2
|
22
|
| 5x Q5 Reaction Buffer
|
20
|
220
|
| Q5 DNA Polymerase
|
1
|
11
|
| **TOTAL**
|
**100**
|
**550**
|
Combined the following for PCR reactions:
* 55μL PCR master mix
* 40μL ligation mix
* 5μL of ILL-BC# (1μM) – The barcode number and the respective sample are listed below.
| **SAMPLE**
|
**BARCODE**
|
**SEQUENCE**
|
| Oly RAD 02
|
1
|
CGTGAT
|
| Oly RAD 03
|
2
|
ACATCG
|
| Oly RAD 04
|
3
|
GCCTAA
|
| Oly RAD 06
|
4
|
TGGTCA
|
| Oly RAD 07
|
5
|
CACTGT
|
| Oly RAD 08
|
6
|
ATTGGC
|
| Oly RAD 14
|
7
|
GATCTG
|
| Oly RAD 17
|
8
|
TCAAGT
|
| Oly RAD 23
|
9
|
CTGATC
|
| Oly RAD 30
|
10
|
AAGCTA
|
Cycling was performed on a PTC-200 (MJ Research) with a heated lid:
| **STEP**
|
**TEMP (C)**
|
**TIME (s)**
|
| Initial Denaturation
|
* 98
|
* 30
|
| 17 cycles
|
* 98
* 60
* 72
|
* 5
* 20
* 10
|
After cycling, added 16μL of 6x loading dye to each sample.
Loaded 10μL of ladder on each of the two gels.
Results:
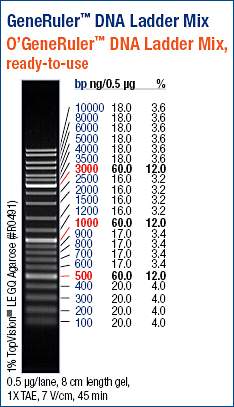(https://raw.githubusercontent.com/sr320/LabDocs/master/protocols/Commercial_Protocols/ThermoFisher_OgeneRuler_DNA_Ladder_Mix_F100439.jpg)
(http://eagle.fish.washington.edu/Arabidopsis/20151113_gel_oly_RAD_prep_PCR_01.png)
(http://eagle.fish.washington.edu/Arabidopsis/20151113_gel_oly_RAD_prep_PCR_02.png)
(http://eagle.fish.washington.edu/Arabidopsis/20151113_gel_oly_RAD_prep_PCR_03.png)
(http://eagle.fish.washington.edu/Arabidopsis/20151113_gel_oly_RAD_prep_PCR_04.png)
Things looked fine. Excised the bands from each sample indicated by the green arrow. Before and after gel images show regions excised. Will purify the bands and quantify library yields.