04-miRNA-comparison
================
Kathleen Durkin
2025-05-05
- 1 Prep data
- 1.1 Isolate mature miRNA
sequences
- 1.2 Check miRNA length
distributions
- 1.3 Merge the three
mature miRNA FASTAs
- 2 BLASTs
- 2.1 Make database for each
species:
- 2.2 Run Blastn
- 2.3 Join
BLAST tables
- 3 Identify conserved miRNAs
- 3.1
Conserved across all three species (Apul, Peve, and Ptuh)
- 3.2 Conserved
among subsets of the three species
- 3.2.1 Apul and
Peve
- 3.2.2 Apul and
Ptuh
- 3.2.3 Peve and
Ptuh
- 3.3 Visualize
- 3.3.1 Data munging of the
results
- 3.3.2 Venn
diagram
- 4 Identify
miRNAs with identical mature miRNAs
- 4.1 Apul
- 4.2 Peve
- 4.3 Ptuh
- 5 Look at the database
matches
- 5.1 Table
I want to find miRNAs that are conserved among either a subset of or all
three species of interest (*A.pulchra*, *P.evermanni*, and
*P.tuahiniensis*) using Blastn. I want to generally investigate sequence
similarity across and within species.
# 1 Prep data
## 1.1 Isolate mature miRNA sequences
Our ShortStack output contains sequences for the mature, star, and
precursor sequences for each identified miRNA. We just want to look at
the mature miRNA sequences right now, so let’s isolate those.
``` bash
cd ../output/04-miRNA-comparison
# Copy all sequences whose headers contain "mature"
awk '/^>/ {p = /mature/} p' ../../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/mir.fasta > Apul_ShortStack_mature.fasta
awk '/^>/ {p = /mature/} p' ../../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta > Peve_ShortStack_mature.fasta
awk '/^>/ {p = /mature/} p' ../../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta > Ptuh_ShortStack_mature.fasta
grep "^>" Apul_ShortStack_mature.fasta | wc -l
grep "^>" Peve_ShortStack_mature.fasta | wc -l
grep "^>" Ptuh_ShortStack_mature.fasta | wc -l
```
39
45
37
## 1.2 Check miRNA length distributions
``` r
# Set our color scheme for plotting -- options for both the abbreviated labels or the full, correct species names
species_colors <- c('A_pulchra' = '#408EC6',
'P_evermanni' = '#1E2761',
'P_tuahiniensis' = '#7A2048')
species_colors_nolabel <- c('#408EC6', '#1E2761', '#7A2048')
```
Apul:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Apul_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Apul_lengths <- read.table("../output/04-miRNA-comparison/Apul_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
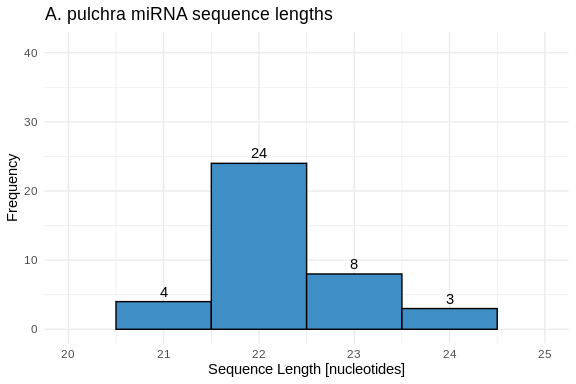
hist_Apul_lengths <- ggplot(Apul_lengths, aes(x = length)) +
geom_histogram(binwidth = 1, fill = species_colors['A_pulchra'], color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "A. pulchra miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20, 25) +
ylim(0, 41) +
theme_minimal()
hist_Apul_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Apul_miRNA_lengths.png",
plot = hist_Apul_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Apul_miRNA_lengths.png",
# plot = hist_Apul_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Ptuh:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Ptuh_lengths <- read.table("../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
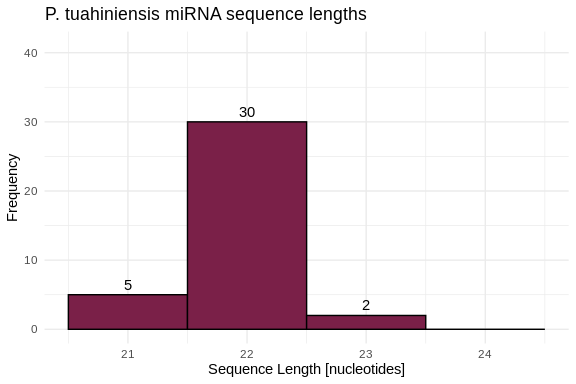
hist_Ptuh_lengths <- ggplot(Ptuh_lengths, aes(x = length)) +
geom_histogram(binwidth = 1, fill = species_colors['P_tuahiniensis'], color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. tuahiniensis miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Ptuh_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Apul_miRNA_lengths.png",
plot = hist_Apul_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Apul_miRNA_lengths.png",
# plot = hist_Apul_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Ptuh:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Ptuh_lengths <- read.table("../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Ptuh_lengths <- ggplot(Ptuh_lengths, aes(x = length)) +
geom_histogram(binwidth = 1, fill = species_colors['P_tuahiniensis'], color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. tuahiniensis miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Ptuh_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Ptuh_miRNA_lengths.png",
plot = hist_Ptuh_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Ptuh_miRNA_lengths.png",
# plot = hist_Ptuh_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Peve:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Peve_lengths <- read.table("../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
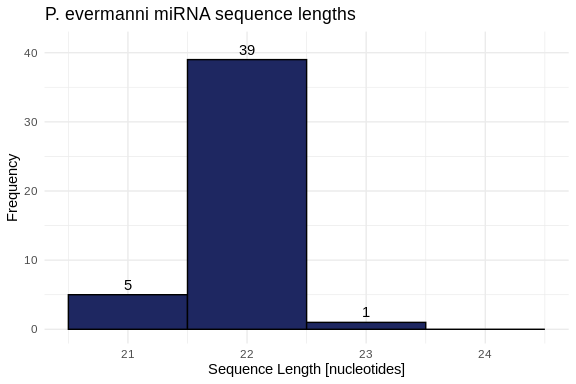
hist_Peve_lengths <- ggplot(Peve_lengths, aes(x = length)) +
geom_histogram(binwidth = 1,
fill = species_colors['P_evermanni'],
color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. evermanni miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Peve_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Ptuh_miRNA_lengths.png",
plot = hist_Ptuh_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Ptuh_miRNA_lengths.png",
# plot = hist_Ptuh_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Peve:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Peve_lengths <- read.table("../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Peve_lengths <- ggplot(Peve_lengths, aes(x = length)) +
geom_histogram(binwidth = 1,
fill = species_colors['P_evermanni'],
color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. evermanni miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Peve_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Peve_miRNA_lengths.png",
plot = hist_Peve_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Peve_miRNA_lengths.png",
# plot = hist_Peve_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
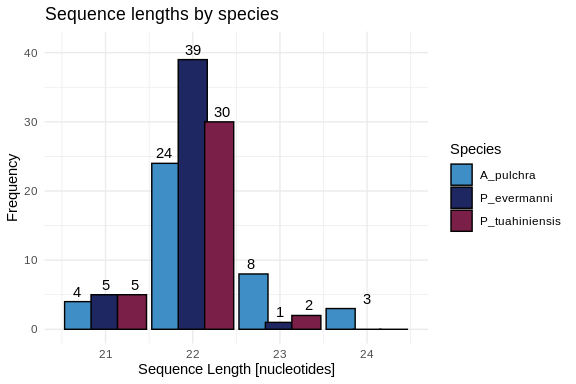
Let’s also make a plot showing the length distributions of all three
species
``` r
# Add a new column to each data frame to label the source file
Apul_lengths <- Apul_lengths %>% mutate(Species = 'A_pulchra')
Peve_lengths <- Peve_lengths %>% mutate(Species = 'P_evermanni')
Ptuh_lengths <- Ptuh_lengths %>% mutate(Species = 'P_tuahiniensis')
# Combine the data frames into one
all_lengths <- rbind(Apul_lengths, Peve_lengths, Ptuh_lengths)
```
``` r
hist_all_lengths <- ggplot(all_lengths, aes(x = length, fill = Species)) +
geom_histogram(binwidth = 1,
position = position_dodge(width = 0.91),
color = "black",
width = 0.9) +
geom_text(stat = 'count',
aes(label = ..count..),
vjust = -0.5,
position = position_dodge(width = 1)) +
scale_fill_manual(values = species_colors) +
labs(title = "Sequence lengths by species",
x = "Sequence Length [nucleotides]",
y = "Frequency",
fill = "Species") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_all_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Peve_miRNA_lengths.png",
plot = hist_Peve_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Peve_miRNA_lengths.png",
# plot = hist_Peve_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Let’s also make a plot showing the length distributions of all three
species
``` r
# Add a new column to each data frame to label the source file
Apul_lengths <- Apul_lengths %>% mutate(Species = 'A_pulchra')
Peve_lengths <- Peve_lengths %>% mutate(Species = 'P_evermanni')
Ptuh_lengths <- Ptuh_lengths %>% mutate(Species = 'P_tuahiniensis')
# Combine the data frames into one
all_lengths <- rbind(Apul_lengths, Peve_lengths, Ptuh_lengths)
```
``` r
hist_all_lengths <- ggplot(all_lengths, aes(x = length, fill = Species)) +
geom_histogram(binwidth = 1,
position = position_dodge(width = 0.91),
color = "black",
width = 0.9) +
geom_text(stat = 'count',
aes(label = ..count..),
vjust = -0.5,
position = position_dodge(width = 1)) +
scale_fill_manual(values = species_colors) +
labs(title = "Sequence lengths by species",
x = "Sequence Length [nucleotides]",
y = "Frequency",
fill = "Species") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_all_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_all_miRNA_lengths.png",
plot = hist_all_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_all_miRNA_lengths.png",
# plot = hist_all_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
``` r
# Summarize min, max, and average lengths for each species
length_summary <- all_lengths %>%
group_by(Species) %>%
summarise(
Min_Length = min(length, na.rm = TRUE),
Max_Length = max(length, na.rm = TRUE),
Avg_Length = mean(length, na.rm = TRUE)
)
print(length_summary)
```
# A tibble: 3 × 4
Species Min_Length Max_Length Avg_Length
1 A_pulchra 21 24 22.3
2 P_evermanni 21 23 21.9
3 P_tuahiniensis 21 23 21.9
``` bash
rm -r ../output/04-miRNA-comparison/*lengths.txt
```
## 1.3 Merge the three mature miRNA FASTAs
``` bash
cd ../output/04-miRNA-comparison
cat Apul_ShortStack_mature.fasta Peve_ShortStack_mature.fasta Ptuh_ShortStack_mature.fasta > merged_all_ShortStack_mature.fasta
head merged_all_ShortStack_mature.fasta
tail merged_all_ShortStack_mature.fasta
```
>Cluster_1826.mature::ntLink_6:4847465-4847486(-)
ATGATCATAGCACTTTCTTTGT
>Cluster_1832.mature::ntLink_6:5157559-5157579(+)
AAATGTTTCGGCTTGTTCCCG
>Cluster_1862.mature::ntLink_6:7263537-7263560(-)
TTTCAAATTAGGAAGGGAGGTGTT
>Cluster_1951.mature::ntLink_6:13351801-13351822(-)
TAAAATGTCGGTTGCTTAAGCT
>Cluster_2463.mature::ptg000001l:5548893-5548914(-)
TCTCAGATTACAGTAGTTAAGT
>Cluster_4823.mature::Pocillopora_meandrina_HIv1___Sc0000018:6855521-6855542(+)
TCACCCAACAGTTTTAATCTGA
>Cluster_5253.mature::Pocillopora_meandrina_HIv1___Sc0000021:4351839-4351860(+)
ACTGATATTCACCAAGTGATTA
>Cluster_5612.mature::Pocillopora_meandrina_HIv1___Sc0000024:4808688-4808708(+)
AGAACCCAAGAATCTCGAAGG
>Cluster_5740.mature::Pocillopora_meandrina_HIv1___Sc0000026:1154772-1154793(-)
TGTACTATGTTCATGATCTTGC
>Cluster_6382.mature::Pocillopora_meandrina_HIv1___Sc0000035:1989842-1989863(+)
TATTTACAACTCTCAAAACAAC
Let’s do a quick investigation of our merged mature miRNAs.
``` bash
# Extract sequence lengths and calculate statistics
lengths=$(awk '/^>/ {if (seqlen) print seqlen; seqlen=0; next} {seqlen += length($0)} END {print seqlen}' ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta)
min_length=$(echo "$lengths" | sort -n | head -n 1)
max_length=$(echo "$lengths" | sort -n | tail -n 1)
total_length=$(echo "$lengths" | awk '{sum += $1} END {print sum}')
num_sequences=$(grep -c '^>' ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta)
average_length=$(echo "scale=2; $total_length / $num_sequences" | bc)
# Output results
echo "Minimum sequence length: $min_length"
echo "Maximum sequence length: $max_length"
echo "Average sequence length: $average_length"
```
Minimum sequence length: 21
Maximum sequence length: 24
Average sequence length: 22.02
# 2 BLASTs
## 2.1 Make database for each species:
Apul
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:49
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 39 sequences in 0.00176692 seconds.
Peve
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:50
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 45 sequences in 0.00195503 seconds.
Ptuh
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:51
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 37 sequences in 0.00155497 seconds.
## 2.2 Run Blastn
Generate a list of blast results that, for each miRNA, shows the top hit
in each of two other species. We want to see the top hits no matter how
poor the match is, so we will not filter by e-value at this stage. We’ll
also include the “-word_size 4” option, which reduces the required
length of the initial match.
All to Apul:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab
All to Peve:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab
All to Ptuh:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab
## 2.3 Join BLAST tables
``` r
apul_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab", sep="\t", header=FALSE)
peve_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab", sep="\t", header=FALSE)
Ptuh_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab", sep="\t", header=FALSE)
```
Column labels: qseqid: Query sequence ID sseqid: Subject (database)
sequence ID pident: Percentage of identical matches length: Alignment
length (number of base pairs or amino acids) mismatch: Number of
mismatches gapopen: Number of gap openings qstart: Start of alignment in
the query qend: End of alignment in the query sstart: Start of alignment
in the subject send: End of alignment in the subject evalue: Expect
value (number of hits expected by chance) bitscore: Bit score
``` r
# Combine the three blast tables
combined_blastn <- rbind(apul_to_all_blastn, peve_to_all_blastn, Ptuh_to_all_blastn)
# Assign informative column labels
colnames(combined_blastn) <- c("qseqid", "sseqid", "pident", "length", "mismatch", "gapopen", "qstart", "qend", "sstart", "send", "evalue", "bitscore")
# Save this original, unfiltered blast table.
write.table(combined_blastn, "../output/04-miRNA-comparison/combined_blast.tab", sep="\t", row.names=FALSE, quote=FALSE)
```
# 3 Identify conserved miRNAs
Filter our list of blast hits to remove instances where sequences match
themselves (e.g. from querying an Apul sequence against our combined
database which contained all Apul sequences), and to retain only the
significant hits (We’ll set this at eval \> 1E-5)
``` r
# Filter
filtered_combined_blastn <- combined_blastn %>%
filter(qseqid != sseqid) %>%
filter(evalue < 0.00001)
# View
nrow(filtered_combined_blastn)
```
[1] 37
``` r
head(filtered_combined_blastn)
```
qseqid
1 Cluster_1167.mature::Porites_evermani_scaffold_49:151640-151661(-)
2 Cluster_5563.mature::Porites_evermani_scaffold_430:205887-205909(-)
3 Cluster_6255.mature::Porites_evermani_scaffold_502:58997-59018(-)
4 Cluster_6914.mature::Porites_evermani_scaffold_594:158230-158250(+)
5 Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)
6 Cluster_8887.mature::Porites_evermani_scaffold_910:118742-118762(+)
sseqid pident length mismatch
1 Cluster_18728.mature::ptg000035l:5346054-5346075(+) 100 22 0
2 Cluster_18772.mature::ptg000035l:8367748-8367770(-) 100 23 0
3 Cluster_3437.mature::ptg000004l:1859911-1859933(-) 100 22 0
4 Cluster_5012.mature::ptg000008l:10754789-10754809(-) 100 21 0
5 Cluster_1832.mature::ntLink_6:5157559-5157579(+) 100 19 0
6 Cluster_1832.mature::ntLink_6:5157559-5157579(+) 100 20 0
gapopen qstart qend sstart send evalue bitscore
1 0 1 22 1 22 5.36e-10 44.1
2 0 1 23 1 23 1.44e-10 46.1
3 0 1 22 1 22 5.36e-10 44.1
4 0 1 21 1 21 1.99e-09 42.1
5 0 2 20 2 20 3.10e-08 38.2
6 0 2 21 2 21 7.85e-09 40.1
``` r
write.table(filtered_combined_blastn, "../output/04-miRNA-comparison/filtered_combined_blast.tab", sep="\t", row.names=FALSE, quote=FALSE)
```
Ok now we can start identifying conserved miRNAs. Keep in mind that this
list of filtered, combined blastn hits contains duplicates because, for
example, querying Apul sequences against a database containing Peve
sequences is functionally the same as querying those Peve sequences
against a databse which contains Apul. So, for example, this list would
contain a hit matching Apul.seq1 to Peve.seq2, *and* a hit matching
Peve.seq2 to Apul.seq1.
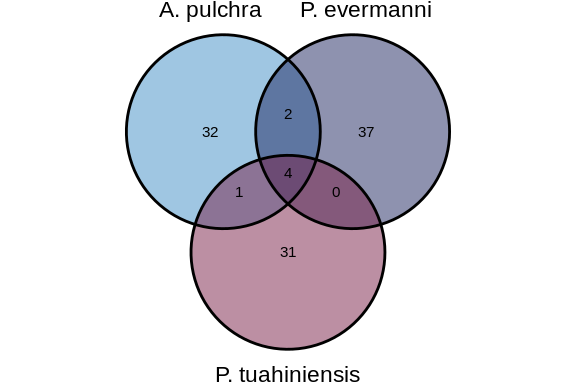
## 3.1 Conserved across all three species (Apul, Peve, and Ptuh)
First, lets find miRNAs conserved among all three species. These would
show up as an miRNA from one species that has hits from both other
species (e.g., Apul.seq1 has a hit from Peve *and* a hit from Ptuh).
``` r
# Find Apul miRNAs that have matches from both Peve and Ptuh
present_in_all <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
filter(any(grepl("Porites_evermani", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match across all three species
# (recall this will include two entries for each conserved miRNA, it's Apul match in Peve, and its Apul match to Ptuh)
head(present_in_all, nrow(present_in_all))
```
# A tibble: 9 × 12
# Groups: sseqid [4]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_1167.… Clust… 100 22 0 0 1 22 1 22
2 Cluster_5563.… Clust… 100 23 0 0 1 23 1 23
3 Cluster_6914.… Clust… 100 21 0 0 1 21 1 21
4 Cluster_14999… Clust… 100 22 0 0 1 22 1 22
5 Cluster_390.m… Clust… 94.7 19 1 0 1 19 1 19
6 Cluster_1116.… Clust… 100 22 0 0 1 22 1 22
7 Cluster_1296.… Clust… 100 21 0 0 2 22 2 22
8 Cluster_1793.… Clust… 100 21 0 0 1 21 1 21
9 Cluster_4039.… Clust… 100 22 0 0 1 22 1 22
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across all three species
paste("Number of miRNAs conserved across all three species:", nrow(distinct(present_in_all, sseqid)))
```
[1] "Number of miRNAs conserved across all three species: 4"
## 3.2 Conserved among subsets of the three species
Now we want to find miRNAs that are conserved withing subsets of the
three species
### 3.2.1 Apul and Peve
Find Apul miRNAs that have hits to Peve miRNAs but *not* hits to Ptuh
miRNAs (that would make them conserved among all three species, which
we’ve already identified)
``` r
# Find Apul miRNAs that have matches from only Peve
present_in_apul_peve <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Peve only
filter(any(grepl("Porites_evermani", qseqid)) & !any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Apul and Peve
head(present_in_apul_peve, nrow(present_in_apul_peve))
```
# A tibble: 4 × 12
# Groups: sseqid [2]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_6255.… Clust… 100 22 0 0 1 22 1 22
2 Cluster_8884.… Clust… 100 19 0 0 2 20 2 20
3 Cluster_8887.… Clust… 100 20 0 0 2 21 2 21
4 Cluster_8888.… Clust… 100 21 0 0 1 21 1 21
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Apul and Peve:", nrow(distinct(present_in_apul_peve, sseqid)))
```
[1] "Number of miRNAs conserved in Apul and Peve: 2"
### 3.2.2 Apul and Ptuh
Find Apul miRNAs that have hits to Ptuh miRNAs but *not* hits to Peve
miRNAs
``` r
# Find Apul miRNAs that have matches from only Ptuh
present_in_apul_Ptuh <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Ptuh only
filter(!any(grepl("Porites_evermani", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Apul and Ptuh
head(present_in_apul_Ptuh, nrow(present_in_apul_Ptuh))
```
# A tibble: 1 × 12
# Groups: sseqid [1]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_1068.… Clust… 100 22 0 0 1 22 1 22
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Apul and Ptuh:", nrow(distinct(present_in_apul_Ptuh, sseqid)))
```
[1] "Number of miRNAs conserved in Apul and Ptuh: 1"
### 3.2.3 Peve and Ptuh
Find Peve miRNAs that have hits to Ptuh miRNAs but *not* hits to Apul
miRNAs
``` r
# Find Peve miRNAs that have matches from only Ptuh
present_in_peve_Ptuh <- filtered_combined_blastn %>%
# isolate Peve miRNAs with hits
filter(grepl("Porites_evermani", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Ptuh only (note the Apul sequence IDs don't contain the species name, so we have to use a non-descriptive unique identifier for filtering)
filter(!any(grepl("mature::ntLink|mature::ptg", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Peve and Ptuh
head(present_in_peve_Ptuh, nrow(present_in_peve_Ptuh))
```
# A tibble: 1 × 12
# Groups: sseqid [1]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send evalue
1 Clust… Clust… 94.7 19 1 0 2 20 3 21 8.54e-6
# ℹ 1 more variable: bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Peve and Ptuh:", nrow(distinct(present_in_peve_Ptuh, sseqid)))
```
[1] "Number of miRNAs conserved in Peve and Ptuh: 1"
## 3.3 Visualize
### 3.3.1 Data munging of the results
``` bash
cd ../output/04-miRNA-comparison
grep "^>" merged_all_ShortStack_mature.fasta | sed 's/^>//' > merged_all_ShortStack_mature_IDs.txt
head -5 merged_all_ShortStack_mature_IDs.txt
```
Cluster_1826.mature::ntLink_6:4847465-4847486(-)
Cluster_1832.mature::ntLink_6:5157559-5157579(+)
Cluster_1862.mature::ntLink_6:7263537-7263560(-)
Cluster_1951.mature::ntLink_6:13351801-13351822(-)
Cluster_2463.mature::ptg000001l:5548893-5548914(-)
``` r
# Read in and separate the ids of all miRNAs from the three species
merged_IDs <- readLines("../output/04-miRNA-comparison/merged_all_ShortStack_mature_IDs.txt")
apul_IDs <- merged_IDs[grep("mature::ntLink|mature::ptg", merged_IDs)]
peve_IDs <- merged_IDs[grep("Porites_evermani", merged_IDs)]
Ptuh_IDs <- merged_IDs[grep("Pocillopora_meandrina", merged_IDs)]
length(apul_IDs)
```
[1] 39
``` r
length(peve_IDs)
```
[1] 45
``` r
length(Ptuh_IDs)
```
[1] 37
``` r
# Assign shared miRNA IDs to conserved miRNAs
# Function to append IDs of matching miRNAs to the original query miRNA
append_IDs <- function(IDs_list, df) {
appended_IDs_list <- vector("list", length(IDs_list))
for (i in seq_along(IDs_list)) {
matching_entries <- df$qseqid[df$sseqid == IDs_list[i]]
if (length(matching_entries) > 0) {
appended_IDs_list[[i]] <- paste(IDs_list[i], paste(matching_entries, collapse = "|"), sep = "|")
} else {
appended_IDs_list[[i]] <- IDs_list[i]
}
}
return(appended_IDs_list)
}
# Apply the function to each set of conserved miRNAs
appendedIDs_apul_peve_Ptuh <- append_IDs(unique(present_in_all$sseqid), present_in_all)
appendedIDs_apul_peve <- append_IDs(unique(present_in_apul_peve$sseqid), present_in_apul_peve)
appendedIDs_apul_Ptuh <- append_IDs(unique(present_in_apul_Ptuh$sseqid), present_in_apul_Ptuh)
appendedIDs_peve_Ptuh <- append_IDs(unique(present_in_peve_Ptuh$sseqid), present_in_peve_Ptuh)
print(appendedIDs_apul_peve_Ptuh[1])
```
[[1]]
[1] "Cluster_18728.mature::ptg000035l:5346054-5346075(+)|Cluster_1167.mature::Porites_evermani_scaffold_49:151640-151661(-)|Cluster_1296.mature::Pocillopora_meandrina_HIv1___Sc0000003:10366055-10366076(+)"
``` r
print(appendedIDs_apul_peve)
```
[[1]]
[1] "Cluster_3437.mature::ptg000004l:1859911-1859933(-)|Cluster_6255.mature::Porites_evermani_scaffold_502:58997-59018(-)"
[[2]]
[1] "Cluster_1832.mature::ntLink_6:5157559-5157579(+)|Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)|Cluster_8887.mature::Porites_evermani_scaffold_910:118742-118762(+)|Cluster_8888.mature::Porites_evermani_scaffold_910:139353-139373(+)"
``` r
print(appendedIDs_apul_Ptuh)
```
[[1]]
[1] "Cluster_17776.mature::ptg000031l:5461327-5461348(-)|Cluster_1068.mature::Pocillopora_meandrina_HIv1___Sc0000002:15749310-15749331(+)"
``` r
print(appendedIDs_peve_Ptuh)
```
[[1]]
[1] "Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)|Cluster_2793.mature::Pocillopora_meandrina_HIv1___Sc0000008:1783824-1783844(+)"
``` r
# combine the new appended IDs into a single list of conserved miRNAs
conserved_miRNAs_all_IDs <- c(appendedIDs_apul_peve_Ptuh, appendedIDs_apul_peve, appendedIDs_apul_Ptuh, appendedIDs_peve_Ptuh)
```
``` r
# For each species list of miRNA IDs, replace species-specific IDs of conserved miRNAs with our newly generated appended IDs. This will created lists of miRNA IDs that have shared IDs for the conserved mRNAs
replace_entries <- function(spec_list, new_conserved_IDs) {
# Iterate over each entry in spec_list
for (i in seq_along(spec_list)) {
# Check if the current entry in spec_list exists in any entry in new_conserved_IDs
matching_entry <- new_conserved_IDs[grep(spec_list[i], new_conserved_IDs, fixed = TRUE)]
# If a match is found, replace the entry in spec_list with the matching entry from new_conserved_IDs
if (length(matching_entry) > 0) {
spec_list[i] <- matching_entry[[1]] # Replace with the first element of matching_entry
}
}
return(spec_list) # Return the modified spec_list
}
apul_mature_newconservedID <- replace_entries(apul_IDs, conserved_miRNAs_all_IDs)
peve_mature_newconservedID <- replace_entries(peve_IDs, conserved_miRNAs_all_IDs)
Ptuh_mature_newconservedID <- replace_entries(Ptuh_IDs, conserved_miRNAs_all_IDs)
```
### 3.3.2 Venn diagram
``` r
# Note that mtORF data indicates our P.tuahiniensis samples are actually P.tuahiniensis, so that's the species name we'll be using in figures
a <- list("A. pulchra" = apul_mature_newconservedID,
"P. evermanni" = peve_mature_newconservedID,
"P. tuahiniensis" = Ptuh_mature_newconservedID)
venn_conserved <- ggvenn(a, show_percentage = FALSE, fill_color = species_colors_nolabel)
venn_conserved
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_all_miRNA_lengths.png",
plot = hist_all_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_all_miRNA_lengths.png",
# plot = hist_all_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
``` r
# Summarize min, max, and average lengths for each species
length_summary <- all_lengths %>%
group_by(Species) %>%
summarise(
Min_Length = min(length, na.rm = TRUE),
Max_Length = max(length, na.rm = TRUE),
Avg_Length = mean(length, na.rm = TRUE)
)
print(length_summary)
```
# A tibble: 3 × 4
Species Min_Length Max_Length Avg_Length
1 A_pulchra 21 24 22.3
2 P_evermanni 21 23 21.9
3 P_tuahiniensis 21 23 21.9
``` bash
rm -r ../output/04-miRNA-comparison/*lengths.txt
```
## 1.3 Merge the three mature miRNA FASTAs
``` bash
cd ../output/04-miRNA-comparison
cat Apul_ShortStack_mature.fasta Peve_ShortStack_mature.fasta Ptuh_ShortStack_mature.fasta > merged_all_ShortStack_mature.fasta
head merged_all_ShortStack_mature.fasta
tail merged_all_ShortStack_mature.fasta
```
>Cluster_1826.mature::ntLink_6:4847465-4847486(-)
ATGATCATAGCACTTTCTTTGT
>Cluster_1832.mature::ntLink_6:5157559-5157579(+)
AAATGTTTCGGCTTGTTCCCG
>Cluster_1862.mature::ntLink_6:7263537-7263560(-)
TTTCAAATTAGGAAGGGAGGTGTT
>Cluster_1951.mature::ntLink_6:13351801-13351822(-)
TAAAATGTCGGTTGCTTAAGCT
>Cluster_2463.mature::ptg000001l:5548893-5548914(-)
TCTCAGATTACAGTAGTTAAGT
>Cluster_4823.mature::Pocillopora_meandrina_HIv1___Sc0000018:6855521-6855542(+)
TCACCCAACAGTTTTAATCTGA
>Cluster_5253.mature::Pocillopora_meandrina_HIv1___Sc0000021:4351839-4351860(+)
ACTGATATTCACCAAGTGATTA
>Cluster_5612.mature::Pocillopora_meandrina_HIv1___Sc0000024:4808688-4808708(+)
AGAACCCAAGAATCTCGAAGG
>Cluster_5740.mature::Pocillopora_meandrina_HIv1___Sc0000026:1154772-1154793(-)
TGTACTATGTTCATGATCTTGC
>Cluster_6382.mature::Pocillopora_meandrina_HIv1___Sc0000035:1989842-1989863(+)
TATTTACAACTCTCAAAACAAC
Let’s do a quick investigation of our merged mature miRNAs.
``` bash
# Extract sequence lengths and calculate statistics
lengths=$(awk '/^>/ {if (seqlen) print seqlen; seqlen=0; next} {seqlen += length($0)} END {print seqlen}' ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta)
min_length=$(echo "$lengths" | sort -n | head -n 1)
max_length=$(echo "$lengths" | sort -n | tail -n 1)
total_length=$(echo "$lengths" | awk '{sum += $1} END {print sum}')
num_sequences=$(grep -c '^>' ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta)
average_length=$(echo "scale=2; $total_length / $num_sequences" | bc)
# Output results
echo "Minimum sequence length: $min_length"
echo "Maximum sequence length: $max_length"
echo "Average sequence length: $average_length"
```
Minimum sequence length: 21
Maximum sequence length: 24
Average sequence length: 22.02
# 2 BLASTs
## 2.1 Make database for each species:
Apul
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:49
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 39 sequences in 0.00176692 seconds.
Peve
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:50
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 45 sequences in 0.00195503 seconds.
Ptuh
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/makeblastdb \
-in ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta \
-dbtype nucl \
-out ../output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
```
Building a new DB, current time: 05/06/2025 01:33:51
New DB name: /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
New DB title: ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta
Sequence type: Nucleotide
Deleted existing Nucleotide BLAST database named /home/shared/8TB_HDD_02/shedurkin/deep-dive-expression/M-multi-species/output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature
Keep MBits: T
Maximum file size: 1000000000B
Adding sequences from FASTA; added 37 sequences in 0.00155497 seconds.
## 2.2 Run Blastn
Generate a list of blast results that, for each miRNA, shows the top hit
in each of two other species. We want to see the top hits no matter how
poor the match is, so we will not filter by e-value at this stage. We’ll
also include the “-word_size 4” option, which reduces the required
length of the initial match.
All to Apul:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Apul-db/Apul_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab
All to Peve:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Peve-db/Peve_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab
All to Ptuh:
``` bash
/home/shared/ncbi-blast-2.11.0+/bin/blastn \
-task blastn-short \
-query ../output/04-miRNA-comparison/merged_all_ShortStack_mature.fasta \
-db ../output/04-miRNA-comparison/blasts/Ptuh-db/Ptuh_ShortStack_mature \
-out ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab \
-num_threads 40 \
-word_size 4 \
-max_target_seqs 1 \
-max_hsps 1 \
-outfmt 6
wc -l ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab
```
Warning: [blastn] Examining 5 or more matches is recommended
121 ../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab
## 2.3 Join BLAST tables
``` r
apul_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Apul_to_all_blastn.tab", sep="\t", header=FALSE)
peve_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Peve_to_all_blastn.tab", sep="\t", header=FALSE)
Ptuh_to_all_blastn <- read.table("../output/04-miRNA-comparison/blasts/Ptuh_to_all_blastn.tab", sep="\t", header=FALSE)
```
Column labels: qseqid: Query sequence ID sseqid: Subject (database)
sequence ID pident: Percentage of identical matches length: Alignment
length (number of base pairs or amino acids) mismatch: Number of
mismatches gapopen: Number of gap openings qstart: Start of alignment in
the query qend: End of alignment in the query sstart: Start of alignment
in the subject send: End of alignment in the subject evalue: Expect
value (number of hits expected by chance) bitscore: Bit score
``` r
# Combine the three blast tables
combined_blastn <- rbind(apul_to_all_blastn, peve_to_all_blastn, Ptuh_to_all_blastn)
# Assign informative column labels
colnames(combined_blastn) <- c("qseqid", "sseqid", "pident", "length", "mismatch", "gapopen", "qstart", "qend", "sstart", "send", "evalue", "bitscore")
# Save this original, unfiltered blast table.
write.table(combined_blastn, "../output/04-miRNA-comparison/combined_blast.tab", sep="\t", row.names=FALSE, quote=FALSE)
```
# 3 Identify conserved miRNAs
Filter our list of blast hits to remove instances where sequences match
themselves (e.g. from querying an Apul sequence against our combined
database which contained all Apul sequences), and to retain only the
significant hits (We’ll set this at eval \> 1E-5)
``` r
# Filter
filtered_combined_blastn <- combined_blastn %>%
filter(qseqid != sseqid) %>%
filter(evalue < 0.00001)
# View
nrow(filtered_combined_blastn)
```
[1] 37
``` r
head(filtered_combined_blastn)
```
qseqid
1 Cluster_1167.mature::Porites_evermani_scaffold_49:151640-151661(-)
2 Cluster_5563.mature::Porites_evermani_scaffold_430:205887-205909(-)
3 Cluster_6255.mature::Porites_evermani_scaffold_502:58997-59018(-)
4 Cluster_6914.mature::Porites_evermani_scaffold_594:158230-158250(+)
5 Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)
6 Cluster_8887.mature::Porites_evermani_scaffold_910:118742-118762(+)
sseqid pident length mismatch
1 Cluster_18728.mature::ptg000035l:5346054-5346075(+) 100 22 0
2 Cluster_18772.mature::ptg000035l:8367748-8367770(-) 100 23 0
3 Cluster_3437.mature::ptg000004l:1859911-1859933(-) 100 22 0
4 Cluster_5012.mature::ptg000008l:10754789-10754809(-) 100 21 0
5 Cluster_1832.mature::ntLink_6:5157559-5157579(+) 100 19 0
6 Cluster_1832.mature::ntLink_6:5157559-5157579(+) 100 20 0
gapopen qstart qend sstart send evalue bitscore
1 0 1 22 1 22 5.36e-10 44.1
2 0 1 23 1 23 1.44e-10 46.1
3 0 1 22 1 22 5.36e-10 44.1
4 0 1 21 1 21 1.99e-09 42.1
5 0 2 20 2 20 3.10e-08 38.2
6 0 2 21 2 21 7.85e-09 40.1
``` r
write.table(filtered_combined_blastn, "../output/04-miRNA-comparison/filtered_combined_blast.tab", sep="\t", row.names=FALSE, quote=FALSE)
```
Ok now we can start identifying conserved miRNAs. Keep in mind that this
list of filtered, combined blastn hits contains duplicates because, for
example, querying Apul sequences against a database containing Peve
sequences is functionally the same as querying those Peve sequences
against a databse which contains Apul. So, for example, this list would
contain a hit matching Apul.seq1 to Peve.seq2, *and* a hit matching
Peve.seq2 to Apul.seq1.
## 3.1 Conserved across all three species (Apul, Peve, and Ptuh)
First, lets find miRNAs conserved among all three species. These would
show up as an miRNA from one species that has hits from both other
species (e.g., Apul.seq1 has a hit from Peve *and* a hit from Ptuh).
``` r
# Find Apul miRNAs that have matches from both Peve and Ptuh
present_in_all <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
filter(any(grepl("Porites_evermani", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match across all three species
# (recall this will include two entries for each conserved miRNA, it's Apul match in Peve, and its Apul match to Ptuh)
head(present_in_all, nrow(present_in_all))
```
# A tibble: 9 × 12
# Groups: sseqid [4]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_1167.… Clust… 100 22 0 0 1 22 1 22
2 Cluster_5563.… Clust… 100 23 0 0 1 23 1 23
3 Cluster_6914.… Clust… 100 21 0 0 1 21 1 21
4 Cluster_14999… Clust… 100 22 0 0 1 22 1 22
5 Cluster_390.m… Clust… 94.7 19 1 0 1 19 1 19
6 Cluster_1116.… Clust… 100 22 0 0 1 22 1 22
7 Cluster_1296.… Clust… 100 21 0 0 2 22 2 22
8 Cluster_1793.… Clust… 100 21 0 0 1 21 1 21
9 Cluster_4039.… Clust… 100 22 0 0 1 22 1 22
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across all three species
paste("Number of miRNAs conserved across all three species:", nrow(distinct(present_in_all, sseqid)))
```
[1] "Number of miRNAs conserved across all three species: 4"
## 3.2 Conserved among subsets of the three species
Now we want to find miRNAs that are conserved withing subsets of the
three species
### 3.2.1 Apul and Peve
Find Apul miRNAs that have hits to Peve miRNAs but *not* hits to Ptuh
miRNAs (that would make them conserved among all three species, which
we’ve already identified)
``` r
# Find Apul miRNAs that have matches from only Peve
present_in_apul_peve <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Peve only
filter(any(grepl("Porites_evermani", qseqid)) & !any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Apul and Peve
head(present_in_apul_peve, nrow(present_in_apul_peve))
```
# A tibble: 4 × 12
# Groups: sseqid [2]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_6255.… Clust… 100 22 0 0 1 22 1 22
2 Cluster_8884.… Clust… 100 19 0 0 2 20 2 20
3 Cluster_8887.… Clust… 100 20 0 0 2 21 2 21
4 Cluster_8888.… Clust… 100 21 0 0 1 21 1 21
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Apul and Peve:", nrow(distinct(present_in_apul_peve, sseqid)))
```
[1] "Number of miRNAs conserved in Apul and Peve: 2"
### 3.2.2 Apul and Ptuh
Find Apul miRNAs that have hits to Ptuh miRNAs but *not* hits to Peve
miRNAs
``` r
# Find Apul miRNAs that have matches from only Ptuh
present_in_apul_Ptuh <- filtered_combined_blastn %>%
# isolate Apul miRNAs with hits
filter(!grepl("Porites_evermani|Pocillopora_meandrina", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Ptuh only
filter(!any(grepl("Porites_evermani", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Apul and Ptuh
head(present_in_apul_Ptuh, nrow(present_in_apul_Ptuh))
```
# A tibble: 1 × 12
# Groups: sseqid [1]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send
1 Cluster_1068.… Clust… 100 22 0 0 1 22 1 22
# ℹ 2 more variables: evalue , bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Apul and Ptuh:", nrow(distinct(present_in_apul_Ptuh, sseqid)))
```
[1] "Number of miRNAs conserved in Apul and Ptuh: 1"
### 3.2.3 Peve and Ptuh
Find Peve miRNAs that have hits to Ptuh miRNAs but *not* hits to Apul
miRNAs
``` r
# Find Peve miRNAs that have matches from only Ptuh
present_in_peve_Ptuh <- filtered_combined_blastn %>%
# isolate Peve miRNAs with hits
filter(grepl("Porites_evermani", sseqid)) %>%
group_by(sseqid) %>%
# filter for hits to Ptuh only (note the Apul sequence IDs don't contain the species name, so we have to use a non-descriptive unique identifier for filtering)
filter(!any(grepl("mature::ntLink|mature::ptg", qseqid)) & any(grepl("Pocillopora_meandrina", qseqid)))
# View the miRNAs that match between Peve and Ptuh
head(present_in_peve_Ptuh, nrow(present_in_peve_Ptuh))
```
# A tibble: 1 × 12
# Groups: sseqid [1]
qseqid sseqid pident length mismatch gapopen qstart qend sstart send evalue
1 Clust… Clust… 94.7 19 1 0 2 20 3 21 8.54e-6
# ℹ 1 more variable: bitscore
``` r
# Count the number of miRNAs conserved across the two species
paste("Number of miRNAs conserved in Peve and Ptuh:", nrow(distinct(present_in_peve_Ptuh, sseqid)))
```
[1] "Number of miRNAs conserved in Peve and Ptuh: 1"
## 3.3 Visualize
### 3.3.1 Data munging of the results
``` bash
cd ../output/04-miRNA-comparison
grep "^>" merged_all_ShortStack_mature.fasta | sed 's/^>//' > merged_all_ShortStack_mature_IDs.txt
head -5 merged_all_ShortStack_mature_IDs.txt
```
Cluster_1826.mature::ntLink_6:4847465-4847486(-)
Cluster_1832.mature::ntLink_6:5157559-5157579(+)
Cluster_1862.mature::ntLink_6:7263537-7263560(-)
Cluster_1951.mature::ntLink_6:13351801-13351822(-)
Cluster_2463.mature::ptg000001l:5548893-5548914(-)
``` r
# Read in and separate the ids of all miRNAs from the three species
merged_IDs <- readLines("../output/04-miRNA-comparison/merged_all_ShortStack_mature_IDs.txt")
apul_IDs <- merged_IDs[grep("mature::ntLink|mature::ptg", merged_IDs)]
peve_IDs <- merged_IDs[grep("Porites_evermani", merged_IDs)]
Ptuh_IDs <- merged_IDs[grep("Pocillopora_meandrina", merged_IDs)]
length(apul_IDs)
```
[1] 39
``` r
length(peve_IDs)
```
[1] 45
``` r
length(Ptuh_IDs)
```
[1] 37
``` r
# Assign shared miRNA IDs to conserved miRNAs
# Function to append IDs of matching miRNAs to the original query miRNA
append_IDs <- function(IDs_list, df) {
appended_IDs_list <- vector("list", length(IDs_list))
for (i in seq_along(IDs_list)) {
matching_entries <- df$qseqid[df$sseqid == IDs_list[i]]
if (length(matching_entries) > 0) {
appended_IDs_list[[i]] <- paste(IDs_list[i], paste(matching_entries, collapse = "|"), sep = "|")
} else {
appended_IDs_list[[i]] <- IDs_list[i]
}
}
return(appended_IDs_list)
}
# Apply the function to each set of conserved miRNAs
appendedIDs_apul_peve_Ptuh <- append_IDs(unique(present_in_all$sseqid), present_in_all)
appendedIDs_apul_peve <- append_IDs(unique(present_in_apul_peve$sseqid), present_in_apul_peve)
appendedIDs_apul_Ptuh <- append_IDs(unique(present_in_apul_Ptuh$sseqid), present_in_apul_Ptuh)
appendedIDs_peve_Ptuh <- append_IDs(unique(present_in_peve_Ptuh$sseqid), present_in_peve_Ptuh)
print(appendedIDs_apul_peve_Ptuh[1])
```
[[1]]
[1] "Cluster_18728.mature::ptg000035l:5346054-5346075(+)|Cluster_1167.mature::Porites_evermani_scaffold_49:151640-151661(-)|Cluster_1296.mature::Pocillopora_meandrina_HIv1___Sc0000003:10366055-10366076(+)"
``` r
print(appendedIDs_apul_peve)
```
[[1]]
[1] "Cluster_3437.mature::ptg000004l:1859911-1859933(-)|Cluster_6255.mature::Porites_evermani_scaffold_502:58997-59018(-)"
[[2]]
[1] "Cluster_1832.mature::ntLink_6:5157559-5157579(+)|Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)|Cluster_8887.mature::Porites_evermani_scaffold_910:118742-118762(+)|Cluster_8888.mature::Porites_evermani_scaffold_910:139353-139373(+)"
``` r
print(appendedIDs_apul_Ptuh)
```
[[1]]
[1] "Cluster_17776.mature::ptg000031l:5461327-5461348(-)|Cluster_1068.mature::Pocillopora_meandrina_HIv1___Sc0000002:15749310-15749331(+)"
``` r
print(appendedIDs_peve_Ptuh)
```
[[1]]
[1] "Cluster_8884.mature::Porites_evermani_scaffold_910:99255-99275(+)|Cluster_2793.mature::Pocillopora_meandrina_HIv1___Sc0000008:1783824-1783844(+)"
``` r
# combine the new appended IDs into a single list of conserved miRNAs
conserved_miRNAs_all_IDs <- c(appendedIDs_apul_peve_Ptuh, appendedIDs_apul_peve, appendedIDs_apul_Ptuh, appendedIDs_peve_Ptuh)
```
``` r
# For each species list of miRNA IDs, replace species-specific IDs of conserved miRNAs with our newly generated appended IDs. This will created lists of miRNA IDs that have shared IDs for the conserved mRNAs
replace_entries <- function(spec_list, new_conserved_IDs) {
# Iterate over each entry in spec_list
for (i in seq_along(spec_list)) {
# Check if the current entry in spec_list exists in any entry in new_conserved_IDs
matching_entry <- new_conserved_IDs[grep(spec_list[i], new_conserved_IDs, fixed = TRUE)]
# If a match is found, replace the entry in spec_list with the matching entry from new_conserved_IDs
if (length(matching_entry) > 0) {
spec_list[i] <- matching_entry[[1]] # Replace with the first element of matching_entry
}
}
return(spec_list) # Return the modified spec_list
}
apul_mature_newconservedID <- replace_entries(apul_IDs, conserved_miRNAs_all_IDs)
peve_mature_newconservedID <- replace_entries(peve_IDs, conserved_miRNAs_all_IDs)
Ptuh_mature_newconservedID <- replace_entries(Ptuh_IDs, conserved_miRNAs_all_IDs)
```
### 3.3.2 Venn diagram
``` r
# Note that mtORF data indicates our P.tuahiniensis samples are actually P.tuahiniensis, so that's the species name we'll be using in figures
a <- list("A. pulchra" = apul_mature_newconservedID,
"P. evermanni" = peve_mature_newconservedID,
"P. tuahiniensis" = Ptuh_mature_newconservedID)
venn_conserved <- ggvenn(a, show_percentage = FALSE, fill_color = species_colors_nolabel)
venn_conserved
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/venn_conserved_miRNA.png",
plot = venn_conserved,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/venn_conserved_miRNA.png",
# plot = venn_conserved,
# res = 600,
# width = 5000,
# height = 5000)
```
# 4 Identify miRNAs with identical mature miRNAs
It’s possible for identical mature miRNAs to arise from non-identical
precursor miRNAs. These would be classified by ShortStack as different
miRNAs, but could still have similar/identical functions. Let’s see if
we have any of those in our data.
We’ve already eliminated instances of miRNAs matching to themselves, so
to identify distinct miRNAs from with identical mature sequences we can
just look for hits within the same species (e.g. Apul.seq1 matching
Apul.seq4)
## 4.1 Apul
``` r
# Identify sets of identical miRNAs
apul_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("mature::ntLink|mature::ptg", sseqid)) %>%
filter(grepl("mature::ntLink|mature::ptg", qseqid))
head(apul_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
``` r
# Save
write.table(apul_identical_miRNAs, "../output/04-miRNA-comparison/Apul_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
There are 0 sets of identical miRNAs identified by ShortStack in
A.pulchra.
## 4.2 Peve
``` r
# Identify sets of identical miRNAs
peve_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Porites_evermani", sseqid)) %>%
filter(grepl("Porites_evermani", qseqid))
head(peve_identical_miRNAs)
```
qseqid
1 Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
sseqid pident
1 Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-) 100
length mismatch gapopen qstart qend sstart send evalue bitscore
1 22 0 0 1 22 1 22 6.06e-10 44.1
``` r
# Save
write.table(peve_identical_miRNAs, "../output/04-miRNA-comparison/Peve_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
``` bash
# First pair
head -2 ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | tail -1
seq1=$(awk 'NR==2 {print $1}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
seq2=$(awk 'NR==2 {print $2}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
echo ""
echo $seq1
echo $seq2
echo ""
# grab the precursor, star, and mature fasta sequences for the two miRNAs
awk -v seq="$seq1" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
awk -v seq="$seq2" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
```
"Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)" "Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)" 100 22 0 0 1 22 1 22 6.06e-10 44.1
Cluster_7657
Cluster_7658
>Cluster_7657::Porites_evermani_scaffold_730:81363-81456(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCGTGGCTCAAACCCTG
>Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7657.star::Porites_evermani_scaffold_730:81414-81436(-)
ACGCTAACTGTAAAACTAACAAG
>Cluster_7658::Porites_evermani_scaffold_730:82401-82494(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCCTGGCTCAAACCCTG
>Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7658.star::Porites_evermani_scaffold_730:82452-82474(-)
ACGCTAACTGTAAAACTAACAAG
For this set of identical miRNAs, the mature and star sequences are
identical and the precursors only have a single mismatch, despite being
located in different places on the chromosome.
## 4.3 Ptuh
``` r
# Identify sets of identical miRNAs
Ptuh_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Pocillopora_meandrina", sseqid)) %>%
filter(grepl("Pocillopora_meandrina", qseqid))
head(Ptuh_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
There are 0 sets of identical miRNAs identified by ShortStack in
P.tuahiniensis
# 5 Look at the database matches
``` bash
# isolate the full "Results" annotation for each mature miRNA, which includes sequence detail and database matches
# Apul
awk -F'\t' 'NR==1 || $20 == "Y"' ../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Apul_results_mature.txt
# Peve
awk -F'\t' 'NR==1 || $20 == "Y"' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Peve_results_mature.txt
# Ptuh
awk -F'\t' 'NR==1 || $20 == "Y"' ../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Ptuh_results_mature.txt
```
Annotate our mature miRNA fasta files with associated database matches
Apul
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Apul_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta > Apul_ShortStack_mature_annotated.fasta
```
Peve
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Peve_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta > Peve_ShortStack_mature_annotated.fasta
```
Ptuh
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Ptuh_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta > Ptuh_ShortStack_mature_annotated.fasta
```
## 5.1 Table
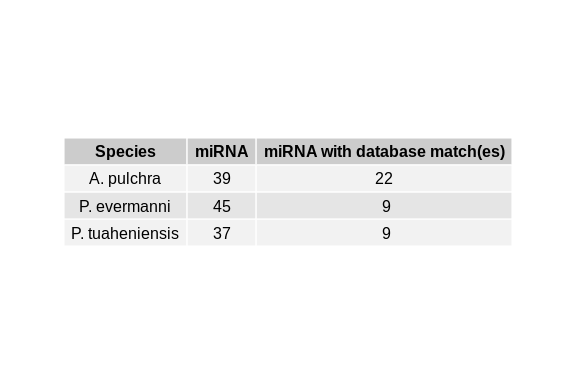
``` r
Apul_shortstack_results <- read.csv("../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt", sep="\t")
Peve_shortstack_results <- read.csv("../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Ptuh_shortstack_results <- read.csv("../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Apul_num_miRNA <- Apul_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Apul_num_miRNAmatch <- Apul_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Peve_num_miRNA <- Peve_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Peve_num_miRNAmatch <- Peve_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Ptuh_num_miRNA <- Ptuh_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Ptuh_num_miRNAmatch <- Ptuh_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
table_data <- data.frame(
Species = c("A. pulchra", "P. evermanni", "P. tuaheniensis"),
miRNA = c(Apul_num_miRNA, Peve_num_miRNA, Ptuh_num_miRNA),
miRNAmatch = c(Apul_num_miRNAmatch, Peve_num_miRNAmatch, Ptuh_num_miRNAmatch)
)
colnames(table_data) <- c("Species", "miRNA", "miRNA with database match(es)")
```
``` r
table <- tableGrob(table_data, rows=NULL)
miRNA_matches_table <- grid.arrange(table)
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/venn_conserved_miRNA.png",
plot = venn_conserved,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/venn_conserved_miRNA.png",
# plot = venn_conserved,
# res = 600,
# width = 5000,
# height = 5000)
```
# 4 Identify miRNAs with identical mature miRNAs
It’s possible for identical mature miRNAs to arise from non-identical
precursor miRNAs. These would be classified by ShortStack as different
miRNAs, but could still have similar/identical functions. Let’s see if
we have any of those in our data.
We’ve already eliminated instances of miRNAs matching to themselves, so
to identify distinct miRNAs from with identical mature sequences we can
just look for hits within the same species (e.g. Apul.seq1 matching
Apul.seq4)
## 4.1 Apul
``` r
# Identify sets of identical miRNAs
apul_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("mature::ntLink|mature::ptg", sseqid)) %>%
filter(grepl("mature::ntLink|mature::ptg", qseqid))
head(apul_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
``` r
# Save
write.table(apul_identical_miRNAs, "../output/04-miRNA-comparison/Apul_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
There are 0 sets of identical miRNAs identified by ShortStack in
A.pulchra.
## 4.2 Peve
``` r
# Identify sets of identical miRNAs
peve_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Porites_evermani", sseqid)) %>%
filter(grepl("Porites_evermani", qseqid))
head(peve_identical_miRNAs)
```
qseqid
1 Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
sseqid pident
1 Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-) 100
length mismatch gapopen qstart qend sstart send evalue bitscore
1 22 0 0 1 22 1 22 6.06e-10 44.1
``` r
# Save
write.table(peve_identical_miRNAs, "../output/04-miRNA-comparison/Peve_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
``` bash
# First pair
head -2 ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | tail -1
seq1=$(awk 'NR==2 {print $1}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
seq2=$(awk 'NR==2 {print $2}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
echo ""
echo $seq1
echo $seq2
echo ""
# grab the precursor, star, and mature fasta sequences for the two miRNAs
awk -v seq="$seq1" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
awk -v seq="$seq2" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
```
"Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)" "Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)" 100 22 0 0 1 22 1 22 6.06e-10 44.1
Cluster_7657
Cluster_7658
>Cluster_7657::Porites_evermani_scaffold_730:81363-81456(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCGTGGCTCAAACCCTG
>Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7657.star::Porites_evermani_scaffold_730:81414-81436(-)
ACGCTAACTGTAAAACTAACAAG
>Cluster_7658::Porites_evermani_scaffold_730:82401-82494(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCCTGGCTCAAACCCTG
>Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7658.star::Porites_evermani_scaffold_730:82452-82474(-)
ACGCTAACTGTAAAACTAACAAG
For this set of identical miRNAs, the mature and star sequences are
identical and the precursors only have a single mismatch, despite being
located in different places on the chromosome.
## 4.3 Ptuh
``` r
# Identify sets of identical miRNAs
Ptuh_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Pocillopora_meandrina", sseqid)) %>%
filter(grepl("Pocillopora_meandrina", qseqid))
head(Ptuh_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
There are 0 sets of identical miRNAs identified by ShortStack in
P.tuahiniensis
# 5 Look at the database matches
``` bash
# isolate the full "Results" annotation for each mature miRNA, which includes sequence detail and database matches
# Apul
awk -F'\t' 'NR==1 || $20 == "Y"' ../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Apul_results_mature.txt
# Peve
awk -F'\t' 'NR==1 || $20 == "Y"' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Peve_results_mature.txt
# Ptuh
awk -F'\t' 'NR==1 || $20 == "Y"' ../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Ptuh_results_mature.txt
```
Annotate our mature miRNA fasta files with associated database matches
Apul
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Apul_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta > Apul_ShortStack_mature_annotated.fasta
```
Peve
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Peve_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta > Peve_ShortStack_mature_annotated.fasta
```
Ptuh
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Ptuh_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta > Ptuh_ShortStack_mature_annotated.fasta
```
## 5.1 Table
``` r
Apul_shortstack_results <- read.csv("../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt", sep="\t")
Peve_shortstack_results <- read.csv("../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Ptuh_shortstack_results <- read.csv("../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Apul_num_miRNA <- Apul_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Apul_num_miRNAmatch <- Apul_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Peve_num_miRNA <- Peve_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Peve_num_miRNAmatch <- Peve_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Ptuh_num_miRNA <- Ptuh_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Ptuh_num_miRNAmatch <- Ptuh_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
table_data <- data.frame(
Species = c("A. pulchra", "P. evermanni", "P. tuaheniensis"),
miRNA = c(Apul_num_miRNA, Peve_num_miRNA, Ptuh_num_miRNA),
miRNAmatch = c(Apul_num_miRNAmatch, Peve_num_miRNAmatch, Ptuh_num_miRNAmatch)
)
colnames(table_data) <- c("Species", "miRNA", "miRNA with database match(es)")
```
``` r
table <- tableGrob(table_data, rows=NULL)
miRNA_matches_table <- grid.arrange(table)
```
 ``` r
png("../output/04-miRNA-comparison/figures/table_miRNA_matches.png", width = 400, height = 100)
grid.arrange(table)
# png("../../supplemental/miRNA/table_miRNA_matches.png", width = 400, height = 100)
# grid.arrange(table)
```
``` r
png("../output/04-miRNA-comparison/figures/table_miRNA_matches.png", width = 400, height = 100)
grid.arrange(table)
# png("../../supplemental/miRNA/table_miRNA_matches.png", width = 400, height = 100)
# grid.arrange(table)
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/venn_conserved_miRNA.png",
plot = venn_conserved,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/venn_conserved_miRNA.png",
# plot = venn_conserved,
# res = 600,
# width = 5000,
# height = 5000)
```
# 4 Identify miRNAs with identical mature miRNAs
It’s possible for identical mature miRNAs to arise from non-identical
precursor miRNAs. These would be classified by ShortStack as different
miRNAs, but could still have similar/identical functions. Let’s see if
we have any of those in our data.
We’ve already eliminated instances of miRNAs matching to themselves, so
to identify distinct miRNAs from with identical mature sequences we can
just look for hits within the same species (e.g. Apul.seq1 matching
Apul.seq4)
## 4.1 Apul
``` r
# Identify sets of identical miRNAs
apul_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("mature::ntLink|mature::ptg", sseqid)) %>%
filter(grepl("mature::ntLink|mature::ptg", qseqid))
head(apul_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
``` r
# Save
write.table(apul_identical_miRNAs, "../output/04-miRNA-comparison/Apul_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
There are 0 sets of identical miRNAs identified by ShortStack in
A.pulchra.
## 4.2 Peve
``` r
# Identify sets of identical miRNAs
peve_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Porites_evermani", sseqid)) %>%
filter(grepl("Porites_evermani", qseqid))
head(peve_identical_miRNAs)
```
qseqid
1 Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
sseqid pident
1 Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-) 100
length mismatch gapopen qstart qend sstart send evalue bitscore
1 22 0 0 1 22 1 22 6.06e-10 44.1
``` r
# Save
write.table(peve_identical_miRNAs, "../output/04-miRNA-comparison/Peve_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
``` bash
# First pair
head -2 ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | tail -1
seq1=$(awk 'NR==2 {print $1}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
seq2=$(awk 'NR==2 {print $2}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
echo ""
echo $seq1
echo $seq2
echo ""
# grab the precursor, star, and mature fasta sequences for the two miRNAs
awk -v seq="$seq1" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
awk -v seq="$seq2" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
```
"Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)" "Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)" 100 22 0 0 1 22 1 22 6.06e-10 44.1
Cluster_7657
Cluster_7658
>Cluster_7657::Porites_evermani_scaffold_730:81363-81456(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCGTGGCTCAAACCCTG
>Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7657.star::Porites_evermani_scaffold_730:81414-81436(-)
ACGCTAACTGTAAAACTAACAAG
>Cluster_7658::Porites_evermani_scaffold_730:82401-82494(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCCTGGCTCAAACCCTG
>Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7658.star::Porites_evermani_scaffold_730:82452-82474(-)
ACGCTAACTGTAAAACTAACAAG
For this set of identical miRNAs, the mature and star sequences are
identical and the precursors only have a single mismatch, despite being
located in different places on the chromosome.
## 4.3 Ptuh
``` r
# Identify sets of identical miRNAs
Ptuh_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Pocillopora_meandrina", sseqid)) %>%
filter(grepl("Pocillopora_meandrina", qseqid))
head(Ptuh_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
There are 0 sets of identical miRNAs identified by ShortStack in
P.tuahiniensis
# 5 Look at the database matches
``` bash
# isolate the full "Results" annotation for each mature miRNA, which includes sequence detail and database matches
# Apul
awk -F'\t' 'NR==1 || $20 == "Y"' ../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Apul_results_mature.txt
# Peve
awk -F'\t' 'NR==1 || $20 == "Y"' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Peve_results_mature.txt
# Ptuh
awk -F'\t' 'NR==1 || $20 == "Y"' ../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Ptuh_results_mature.txt
```
Annotate our mature miRNA fasta files with associated database matches
Apul
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Apul_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta > Apul_ShortStack_mature_annotated.fasta
```
Peve
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Peve_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta > Peve_ShortStack_mature_annotated.fasta
```
Ptuh
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Ptuh_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta > Ptuh_ShortStack_mature_annotated.fasta
```
## 5.1 Table
``` r
Apul_shortstack_results <- read.csv("../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt", sep="\t")
Peve_shortstack_results <- read.csv("../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Ptuh_shortstack_results <- read.csv("../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Apul_num_miRNA <- Apul_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Apul_num_miRNAmatch <- Apul_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Peve_num_miRNA <- Peve_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Peve_num_miRNAmatch <- Peve_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Ptuh_num_miRNA <- Ptuh_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Ptuh_num_miRNAmatch <- Ptuh_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
table_data <- data.frame(
Species = c("A. pulchra", "P. evermanni", "P. tuaheniensis"),
miRNA = c(Apul_num_miRNA, Peve_num_miRNA, Ptuh_num_miRNA),
miRNAmatch = c(Apul_num_miRNAmatch, Peve_num_miRNAmatch, Ptuh_num_miRNAmatch)
)
colnames(table_data) <- c("Species", "miRNA", "miRNA with database match(es)")
```
``` r
table <- tableGrob(table_data, rows=NULL)
miRNA_matches_table <- grid.arrange(table)
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/venn_conserved_miRNA.png",
plot = venn_conserved,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/venn_conserved_miRNA.png",
# plot = venn_conserved,
# res = 600,
# width = 5000,
# height = 5000)
```
# 4 Identify miRNAs with identical mature miRNAs
It’s possible for identical mature miRNAs to arise from non-identical
precursor miRNAs. These would be classified by ShortStack as different
miRNAs, but could still have similar/identical functions. Let’s see if
we have any of those in our data.
We’ve already eliminated instances of miRNAs matching to themselves, so
to identify distinct miRNAs from with identical mature sequences we can
just look for hits within the same species (e.g. Apul.seq1 matching
Apul.seq4)
## 4.1 Apul
``` r
# Identify sets of identical miRNAs
apul_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("mature::ntLink|mature::ptg", sseqid)) %>%
filter(grepl("mature::ntLink|mature::ptg", qseqid))
head(apul_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
``` r
# Save
write.table(apul_identical_miRNAs, "../output/04-miRNA-comparison/Apul_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
There are 0 sets of identical miRNAs identified by ShortStack in
A.pulchra.
## 4.2 Peve
``` r
# Identify sets of identical miRNAs
peve_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Porites_evermani", sseqid)) %>%
filter(grepl("Porites_evermani", qseqid))
head(peve_identical_miRNAs)
```
qseqid
1 Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
sseqid pident
1 Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-) 100
length mismatch gapopen qstart qend sstart send evalue bitscore
1 22 0 0 1 22 1 22 6.06e-10 44.1
``` r
# Save
write.table(peve_identical_miRNAs, "../output/04-miRNA-comparison/Peve_identical_miRNAs.tab", sep="\t", row.names = FALSE, col.names = TRUE)
```
``` bash
# First pair
head -2 ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | tail -1
seq1=$(awk 'NR==2 {print $1}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
seq2=$(awk 'NR==2 {print $2}' FS='\t' ../output/04-miRNA-comparison/Peve_identical_miRNAs.tab | sed 's/.mature::.*//' | sed 's/"//g')
echo ""
echo $seq1
echo $seq2
echo ""
# grab the precursor, star, and mature fasta sequences for the two miRNAs
awk -v seq="$seq1" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
awk -v seq="$seq2" 'BEGIN {RS=">"; FS="\n"} $1 ~ seq {print ">"$0}' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/mir.fasta
```
"Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)" "Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)" 100 22 0 0 1 22 1 22 6.06e-10 44.1
Cluster_7657
Cluster_7658
>Cluster_7657::Porites_evermani_scaffold_730:81363-81456(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCGTGGCTCAAACCCTG
>Cluster_7657.mature::Porites_evermani_scaffold_730:81385-81406(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7657.star::Porites_evermani_scaffold_730:81414-81436(-)
ACGCTAACTGTAAAACTAACAAG
>Cluster_7658::Porites_evermani_scaffold_730:82401-82494(-)
TCAACAGTAAAACTAACAAAACGCTAACTGTAAAACTAACAAGTTCAACTTGTTAGTTTA
CAGTTAGTGTTTTGTTAGCCTGGCTCAAACCCTG
>Cluster_7658.mature::Porites_evermani_scaffold_730:82423-82444(-)
TGTTAGTTTACAGTTAGTGTTT
>Cluster_7658.star::Porites_evermani_scaffold_730:82452-82474(-)
ACGCTAACTGTAAAACTAACAAG
For this set of identical miRNAs, the mature and star sequences are
identical and the precursors only have a single mismatch, despite being
located in different places on the chromosome.
## 4.3 Ptuh
``` r
# Identify sets of identical miRNAs
Ptuh_identical_miRNAs <- filtered_combined_blastn %>%
filter(grepl("Pocillopora_meandrina", sseqid)) %>%
filter(grepl("Pocillopora_meandrina", qseqid))
head(Ptuh_identical_miRNAs)
```
[1] qseqid sseqid pident length mismatch gapopen qstart qend
[9] sstart send evalue bitscore
<0 rows> (or 0-length row.names)
There are 0 sets of identical miRNAs identified by ShortStack in
P.tuahiniensis
# 5 Look at the database matches
``` bash
# isolate the full "Results" annotation for each mature miRNA, which includes sequence detail and database matches
# Apul
awk -F'\t' 'NR==1 || $20 == "Y"' ../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Apul_results_mature.txt
# Peve
awk -F'\t' 'NR==1 || $20 == "Y"' ../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Peve_results_mature.txt
# Ptuh
awk -F'\t' 'NR==1 || $20 == "Y"' ../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt > ../output/04-miRNA-comparison/Ptuh_results_mature.txt
```
Annotate our mature miRNA fasta files with associated database matches
Apul
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Apul_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Apul_ShortStack_mature.fasta > Apul_ShortStack_mature_annotated.fasta
```
Peve
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Peve_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta > Peve_ShortStack_mature_annotated.fasta
```
Ptuh
``` bash
cd ../output/04-miRNA-comparison
while IFS= read -r line; do
if [[ $line == ">"* ]]; then
# Extract sequence header (without ">") and the accession string
header="${line:1}"
accession=$(echo "$header" | awk -F '.' '{print $1}')
# Search for the accession string in the metadata file
metadata_line=$(grep -i "$accession" Ptuh_results_mature.txt)
# Extract the last column entry from the metadata line
last_column=$(echo "$metadata_line" | awk '{print $NF}')
# Append the last column entry to the sequence header
new_header="$header $last_column"
# Output the new header
echo ">$new_header"
else
# Output the sequence line unchanged
echo "$line"
fi
done < ../../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta > Ptuh_ShortStack_mature_annotated.fasta
```
## 5.1 Table
``` r
Apul_shortstack_results <- read.csv("../../D-Apul/output/11-Apul-sRNA-ShortStack_4.1.0-pulchra_genome/ShortStack_out/Results.txt", sep="\t")
Peve_shortstack_results <- read.csv("../../E-Peve/output/05-Peve-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Ptuh_shortstack_results <- read.csv("../../F-Ptuh/output/05-Ptuh-sRNA-ShortStack_4.1.0/ShortStack_out/Results.txt", sep="\t")
Apul_num_miRNA <- Apul_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Apul_num_miRNAmatch <- Apul_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Peve_num_miRNA <- Peve_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Peve_num_miRNAmatch <- Peve_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
Ptuh_num_miRNA <- Ptuh_shortstack_results %>% filter(MIRNA == "Y") %>% nrow()
Ptuh_num_miRNAmatch <- Ptuh_shortstack_results %>% filter(MIRNA == "Y" ) %>% filter(!is.na(known_miRNAs)) %>% nrow()
table_data <- data.frame(
Species = c("A. pulchra", "P. evermanni", "P. tuaheniensis"),
miRNA = c(Apul_num_miRNA, Peve_num_miRNA, Ptuh_num_miRNA),
miRNAmatch = c(Apul_num_miRNAmatch, Peve_num_miRNAmatch, Ptuh_num_miRNAmatch)
)
colnames(table_data) <- c("Species", "miRNA", "miRNA with database match(es)")
```
``` r
table <- tableGrob(table_data, rows=NULL)
miRNA_matches_table <- grid.arrange(table)
```
 ``` r
png("../output/04-miRNA-comparison/figures/table_miRNA_matches.png", width = 400, height = 100)
grid.arrange(table)
# png("../../supplemental/miRNA/table_miRNA_matches.png", width = 400, height = 100)
# grid.arrange(table)
```
``` r
png("../output/04-miRNA-comparison/figures/table_miRNA_matches.png", width = 400, height = 100)
grid.arrange(table)
# png("../../supplemental/miRNA/table_miRNA_matches.png", width = 400, height = 100)
# grid.arrange(table)
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Apul_miRNA_lengths.png",
plot = hist_Apul_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Apul_miRNA_lengths.png",
# plot = hist_Apul_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Ptuh:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Ptuh_lengths <- read.table("../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Ptuh_lengths <- ggplot(Ptuh_lengths, aes(x = length)) +
geom_histogram(binwidth = 1, fill = species_colors['P_tuahiniensis'], color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. tuahiniensis miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Ptuh_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Apul_miRNA_lengths.png",
plot = hist_Apul_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Apul_miRNA_lengths.png",
# plot = hist_Apul_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Ptuh:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Ptuh_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Ptuh_lengths <- read.table("../output/04-miRNA-comparison/Ptuh_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Ptuh_lengths <- ggplot(Ptuh_lengths, aes(x = length)) +
geom_histogram(binwidth = 1, fill = species_colors['P_tuahiniensis'], color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. tuahiniensis miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Ptuh_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Ptuh_miRNA_lengths.png",
plot = hist_Ptuh_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Ptuh_miRNA_lengths.png",
# plot = hist_Ptuh_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Peve:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Peve_lengths <- read.table("../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Peve_lengths <- ggplot(Peve_lengths, aes(x = length)) +
geom_histogram(binwidth = 1,
fill = species_colors['P_evermanni'],
color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. evermanni miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Peve_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Ptuh_miRNA_lengths.png",
plot = hist_Ptuh_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Ptuh_miRNA_lengths.png",
# plot = hist_Ptuh_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Peve:
``` bash
# Extract sequence lengths and calculate statistics
grep -v '^>' ../output/04-miRNA-comparison/Peve_ShortStack_mature.fasta | awk '{ print length($0) }' > ../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt
```
``` r
# Read in the lengths
Peve_lengths <- read.table("../output/04-miRNA-comparison/Peve_ShortStack_mature_lengths.txt", header = FALSE, col.names = "length")
# Make histogram of lengths
hist_Peve_lengths <- ggplot(Peve_lengths, aes(x = length)) +
geom_histogram(binwidth = 1,
fill = species_colors['P_evermanni'],
color = "black") +
geom_text(stat = 'count', aes(label = ..count..), vjust = -0.5) +
labs(title = "P. evermanni miRNA sequence lengths",
x = "Sequence Length [nucleotides]",
y = "Frequency") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_Peve_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Peve_miRNA_lengths.png",
plot = hist_Peve_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Peve_miRNA_lengths.png",
# plot = hist_Peve_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Let’s also make a plot showing the length distributions of all three
species
``` r
# Add a new column to each data frame to label the source file
Apul_lengths <- Apul_lengths %>% mutate(Species = 'A_pulchra')
Peve_lengths <- Peve_lengths %>% mutate(Species = 'P_evermanni')
Ptuh_lengths <- Ptuh_lengths %>% mutate(Species = 'P_tuahiniensis')
# Combine the data frames into one
all_lengths <- rbind(Apul_lengths, Peve_lengths, Ptuh_lengths)
```
``` r
hist_all_lengths <- ggplot(all_lengths, aes(x = length, fill = Species)) +
geom_histogram(binwidth = 1,
position = position_dodge(width = 0.91),
color = "black",
width = 0.9) +
geom_text(stat = 'count',
aes(label = ..count..),
vjust = -0.5,
position = position_dodge(width = 1)) +
scale_fill_manual(values = species_colors) +
labs(title = "Sequence lengths by species",
x = "Sequence Length [nucleotides]",
y = "Frequency",
fill = "Species") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_all_lengths
```
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_Peve_miRNA_lengths.png",
plot = hist_Peve_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_Peve_miRNA_lengths.png",
# plot = hist_Peve_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
Let’s also make a plot showing the length distributions of all three
species
``` r
# Add a new column to each data frame to label the source file
Apul_lengths <- Apul_lengths %>% mutate(Species = 'A_pulchra')
Peve_lengths <- Peve_lengths %>% mutate(Species = 'P_evermanni')
Ptuh_lengths <- Ptuh_lengths %>% mutate(Species = 'P_tuahiniensis')
# Combine the data frames into one
all_lengths <- rbind(Apul_lengths, Peve_lengths, Ptuh_lengths)
```
``` r
hist_all_lengths <- ggplot(all_lengths, aes(x = length, fill = Species)) +
geom_histogram(binwidth = 1,
position = position_dodge(width = 0.91),
color = "black",
width = 0.9) +
geom_text(stat = 'count',
aes(label = ..count..),
vjust = -0.5,
position = position_dodge(width = 1)) +
scale_fill_manual(values = species_colors) +
labs(title = "Sequence lengths by species",
x = "Sequence Length [nucleotides]",
y = "Frequency",
fill = "Species") +
xlim(20.5, 24.5) +
ylim(0, 41) +
theme_minimal()
hist_all_lengths
```
 ``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_all_miRNA_lengths.png",
plot = hist_all_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_all_miRNA_lengths.png",
# plot = hist_all_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
``` r
# Summarize min, max, and average lengths for each species
length_summary <- all_lengths %>%
group_by(Species) %>%
summarise(
Min_Length = min(length, na.rm = TRUE),
Max_Length = max(length, na.rm = TRUE),
Avg_Length = mean(length, na.rm = TRUE)
)
print(length_summary)
```
# A tibble: 3 × 4
Species Min_Length Max_Length Avg_Length
``` r
ggexport(filename = "../output/04-miRNA-comparison/figures/histogram_all_miRNA_lengths.png",
plot = hist_all_lengths,
res = 600,
width = 5000,
height = 5000)
# ggexport(filename = "../../supplemental/miRNA/histogram_all_miRNA_lengths.png",
# plot = hist_all_lengths,
# res = 600,
# width = 5000,
# height = 5000)
```
``` r
# Summarize min, max, and average lengths for each species
length_summary <- all_lengths %>%
group_by(Species) %>%
summarise(
Min_Length = min(length, na.rm = TRUE),
Max_Length = max(length, na.rm = TRUE),
Avg_Length = mean(length, na.rm = TRUE)
)
print(length_summary)
```
# A tibble: 3 × 4
Species Min_Length Max_Length Avg_Length